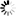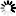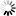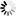Start taking cold showers daily?
Below is a text from a link to newscientist.com from Wim Hof's Twitter page.
"Cold exposure stops tumour growth in mice by hijacking glucose stores
Being continuously exposed to 4°C (39°F) for 20 days inhibited tumour growth and improved the survival of mice with five different types of cancerous cells
A brown fat cell, surrounded by capillaries, captured via a coloured scanning electron micrograph. Activating brown fat through cold exposure may cause it to burn glucose, which cancerous tumour cells rely on for growth
Cold temperatures could inhibit the growth of cancerous cells, according to research conducted in mice and one person with Hodgkin’s lymphoma.
Exposure to low temperatures is thought to prompt brown fat cells to burn glucose, cancer cells’ dominant energy source.
Some existing cancer treatments disrupt glucose uptake to slow or prevent tumour growth. These tend to be administered via drugs, rather than whole-body exposure to cold therapy. Drugs may have more side effects and complex administration regimens, relative to cold therapy.
To test cold therapy’s potential, Yihai Cao at the Karolinska Institute in Sweden and his colleagues implanted five different types of cancerous cells into a group of mice. Some of the rodents were then continuously exposed to very low temperatures, but above freezing, for 20 days.
This exposure activated the mice’s brown fat tissue, which burns energy rather than storing it, reducing the tumours’ energy supply.
These mice went on to have considerable tumour inhibition and a survival rate that was almost double that of mice that received no treatment.
“This is really something new: not directly targeting cancer cells, but rather altering global metabolism in the body to affect tumours,” says Cao.
To eliminate the possibility that the tumour suppression was down to something other than the cold therapy, Cao and his team intervened in several ways. After surgically removing the cold-exposed mice’s brown fat, or turning off the gene by which brown fat generates heat, tumour inhibition was absent.
The inhibition was also absent when the mice were fed a high-glucose diet, suggesting that the tumours’ growth was inhibited by a lack of glucose. The team also performed a genetic analysis on the cold-exposed tumours, finding a decrease in markers associated with glucose consumption.
In a second part of the experiment, Cao and his team exposed a group of six healthy human volunteers to 16°C (61°F) for 2 to 6 hours a day for two weeks. Similarly to the mice, the volunteers’ brown fat tissue became activated.
The researchers then continuously exposed a person with Hodgkin’s lymphoma to 22°C (72°F) for seven days. Not only did their brown fat become activated, their tumours also consumed less glucose over this period.
The results are solid, but more research needs to be carried out, both in animals and in humans with tumours, says Katiuscia Bianchi at the Barts Cancer Institute in London.
According to Saverio Tardito at the Beatson Institute in Glasgow, UK, the mice tumour growth results are “striking”. But given that this treatment would most likely be administered in combination with chemotherapy, it might negatively affect chemotherapy drugs, he says, which has to be ruled out before any joint treatments are carried out.
Cao himself concedes that the genetic variation in people’s brown fat and its response to cold might make the treatment unsuitable for some. People with later-stage cancer who have already lost weight also may be unable to risk losing more through brown fat-driven energy consumption, he says."
Journal reference: Nature, DOI: 10.1038/s41586-022-05030-3